8 Things You Need to Know About Anodizing Aluminum
Anodizing is an electrochemical process for increasing the thickness of natural metal oxide layer for durability and additional protection against corrosion. Anodizing also offers scratch resistance and improves surface finish and gives it an aesthetic look. It can further be coated with a color dye. Anodizing can be done on a range of materials but aluminum is most commonly anodized. Let’s understand the process and considerations.

1. Process of Anodizing Aluminum
The aluminum part is first cleaned and rinsed thoroughly and placed in electrolytic bath like dilute sulfuric acid or dilute nitric acid. Acid increases the electrical conductivity of water hence making the process faster. Then electricity is passed through the electrolyte. Positive charge is applied to the aluminum part under consideration and negative charge is applied to the plate suspended in the electrolyte. The water in the electrolyte gets split into positive hydrogen ions at the cathode and negative oxygen ions at the anode. Negative oxygen gets in contact with the aluminum atoms on the surface and gets converted into aluminum oxide.
2. Barrier Layer in Anodizing
The oxide layer formed on the surface of aluminum is stable making it resistant to further chemical reactions or corrosion. Hence the first layer formed on the surface is called a barrier layer.
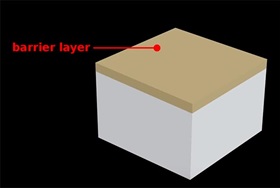
The barrier layer protects against further oxidation at the surface
The structure of Aluminum oxide has tiny pores on it. If the current is passed through a controlled duration, the reactive pores continue to grow forming column like structures.
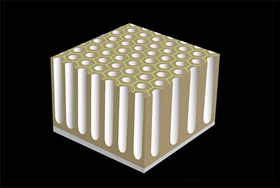
A regular pattern of surface porosity is created when electric current is applied.
The longer the current is applied the greater the penetration of these columns. For typical non-hard coatings, the depth can be up to 10 microns which is sufficient to withstand chemical attack and also is very scratch resistant. At this stage the part can be removed and rinsed with water to leave it as is or can be dye coated to give it a perfect shiny metallic color.
3. Hard Anodizing
Hard anodizing, sometimes called Type III, offers greater corrosion protection and resistance to wear in extreme environments or with moving mechanical parts subject to a lot of friction. This is produced by continuing the electrical current until the depth of the pores is 25 microns or even more. This takes more time and is more expensive but produces a superior result.
4. Does Aluminum Need Corrosion Protection?
Corrosion and rusting happens when metal reacts with oxygen to form metal oxides. Although aluminum oxide is stable preventing further reaction with oxygen, it still needs to be protected against other environments like acids, salts and other contaminants
5. Adding Color
Stable pores on the anodized aluminum pose perfect suitability to etch with colored dyes and pigments. Hence the ability of anodizing that creates surface pores utilized to color the surface. Hence anodizing has become almost synonymous with adding color.
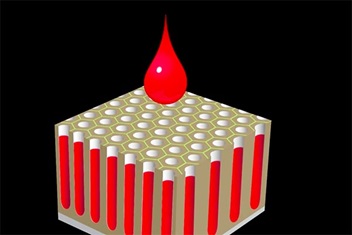
Empty pores make it ideal for adding colorants.
The pigment fills all the empty pores up to the surface, where it’s then permanently sealed off. That’s why anodized colors are so durable – they can’t be scratched off from the surface easily although grinding away the substrate can remove the coat.
6. Characteristic Metallic Sheen
Due to uniform electro-chemical etching leaving a rough surface, anodized aluminum gives a metallic look. When color is added, the deep pores reflect light waves that interact with the ones coming from the pores and the ones coming from the surface, thus giving it a distinctive shine.

Light changes colors as it reflects from an anodized surface.
So the light that bounces back to strike your eye will in fact be a combination of two distinct wavelengths interacting as they reflect from slightly different surfaces. This causes the distinctive shine of aluminum anodizing.
7. Anodizing Other Metals
Anodizing also works with magnesium, titanium and even conductive plastics. It’s inexpensive, reliable and eminently durable. That’s why it’s so commonly used in architectural fittings, because it’s both beautiful and almost impervious to the effects of weathering.
8. Anodize an Entire Part
As the aluminum part needs to be hanged in the electrolyte, some portion of it that is in contact with the hanger does not get exposed. Hence either the part is hung in different stages of electrolyte baths with varying positions or the orientation is chosen such that the vital portion for aesthetic look is properly exposed.